Can Damage Engines
A Brief History of Aircraft Carburetors and Fuel Systems
Part 3: Aviation Fuels
by Terry Welshans
Bardstown, Kentucky
for the Aircraft Engine Historical Society
August 2013
Table of Contents
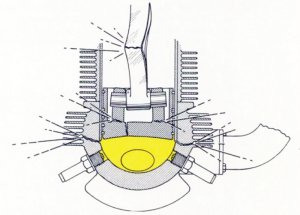
Fuels for use in internal combustion engines must be either a gas, or a liquid that has evaporated into a gas. Any liquid entering the combustion chamber can cause a catastrophic engine failure if the volume of liquid in the cylinders more than the volume of the combustion chamber when the piston is at the top of its stroke, as liquids are not normally compressible. As the engine crankshaft rotates, the piston cannot continue moving toward the cylinder head,resulting in a hydraulic lock. This causes the connecting rod to bend or break as the distance between the piston and crankshaft continues reducing as the crankshaft continues rotating.[60]
 |
| Fig. 10. Liquid in Combustion Chambers Can Damage Engines |
Gasoline is the fuel of choice for internal combustion engines. Although it is a liquid at room temperature, it boils, producing a vapor, at a reasonably low temperature. Heat from a running engine is sufficiently hot to evaporate the gasoline in the event some liquid fuel makes it into the intake manifold. When the engine is cold, one must take care to prevent excess fuel entering the engine as the engine starts, a condition commonly known as "flooding" the engine. Gasolines used in early engines was not consistent from day to day, and were of generally poor quality. Compared to the gasoline produced today, it had a low octane rating that varied from 40 to 70 at best. Today, the octane rating of a fuel helps the user find the correct gasoline as required by the engine maker. In the early days, no one knew about such things. Once automobile and engine builders discovered that they could build race cars, development of fast race cars became a matter of pride, and competition led to more powerful engines, and better fuels.
James Allison opened an engine shop in Indianapolis, Indiana that catered to the racers, and his contributions led to great fame and fortune in car racing circles.[61] His shop could tackle almost any engine related problem, and horsepower became the number one priority. One experiment found that a fast way to improve an engine’s power was to increase the compression ratio, which is the ratio between the two volumes of one engine cylinder and that of the volume its combustion chamber, including the volume above the top of the piston. High compression ratios resulted in new, strange noises within the engine. Called "engine knock" at the time, experiments found that the problem was from the spontaneous ignition of the fuel. The next project for the engineers was to find a way to prevent the knock, and that was no small job, as the chemistry of gasoline was then in its infancy. One of the first discoveries was that lead compounds added to the fuel reduced the knock, and that mixing lead compounds into gasoline could make a useable high performance fuel suitable for high performance engines.
Gasoline is not a simple single chemical. It is composed of a mixture of hundreds of chemicals with the portions of each one varying almost randomly. As chemistry advanced, gasoline became ripe for development, as it had to keep pace with engine development.
Many of the words used to name experimental fuels are quite complicated, even in their simplest form. These names are composed of a few basic terms that are very descriptive, once understood. There is no intention here to make the reader into a chemistry graduate student; rather it is best to make the reader more competent in understanding the terminology of the fuel industry, particularly the complex fuel constituents that allowed aviation fuels to produce the desired power output for the high performance fighters and bombers of World War II.
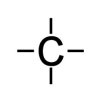
First, a few terms that the reader will frequently encounter later in this article. Catenation is the linkage of atoms of the same element into longer chains called polymers. Catenation occurs most readily in carbon, which forms covalent[62] (double) bonds with other carbon atoms to create longer chains and structures called catenae.[63] An alkane is a cyclic (repeating) branched or un-branched hydrocarbon having the general formula CnH(2n + 2) (where n is any number), that consist entirely of hydrogen atoms and saturated carbon atoms. Saturated atoms are not able to easily decompose or combine with other atoms as their outermost electron ring (or valence ring) has no empty positions for other electrons of other atoms to occupy, as that is how atoms connect to one another in chemical compounds. Carbon atoms have four open positions available in the valence ring that other atoms can join or share. Any atom can join carbon. A carbon atom can join to another carbon atom with up to three bonds, making a very strong connection.
 |
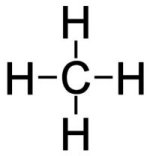 |
| Fig. 11. Carbon Atom Structure. In this Lewis-style representation, four lines that represent four open valence positions. If all four of a carbon atom’s open positions have a hydrogen atom attached, the carbon atom is then saturated, and nothing else can join to it. | Fig. 12. Hydrogen-Saturated Carbon Atom Structure. Hydrogen has only one open position in its valence ring, allowing carbon to have up to four hydrogen atoms attached to it. As only one bond is possible to the carbon atom, the connection is not very strong, making hydrocarbons excellent fuels. |
Alkanes
Straight-chain alkanes[64] take the suffix "ane" and are prefixed depending on the number of carbon atoms in the chain, following a set of standard rules.
| Number of carbon atoms | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Prefix | Meth | Eth | Prop | But | Pent | Hex | Hept | Oct | Non | Dec | Undec | Codec | Trizec |
Taking the prefix "meth" from a molecule with one carbon atom, and then adding the "ane" suffix, the resulting chemical name is "methane." Another example, the nine-carbon alkane CH3(CH2)7CH3, the resulting chemical name is "nonane." The names of the first four alkanes are from methanol, ether, propionic acid and butyric acid, respectively. The remaining alkanes have a Greek numeric prefix, with the exceptions of nonane, which has a Latin prefix.
Cycloalkane
Cycloalkanes (also called naphthenes) are types of alkanes that have one or more rings of carbon atoms in the chemical structure of their molecules.[65] Alkanes are types of organic hydrocarbon compounds that have only single chemical bonds in their chemical structure. Cycloalkanes consist of only carbon (C) and hydrogen (H) atoms, saturated with no multiple C-C bonds to hydrogenate (add more hydrogen to). Cyclic alkanes have prefixes with "cyclo-": for example, C4H8 is cyclobutane and C6H12 is cyclohexane.
| Number of Carbon Atoms | Alkane (single bond) | Alkene (double bond) | Alkyne (triple bond) | Cycloalkane | Alkadiene |
|---|---|---|---|---|---|
| 1 | Methane | Methene | Methyne | --- | --- |
| 2 | Ethane | Ethene (ethylene) | Ethyne(acetylene) | --- | --- |
| 3 | Propane | Propene (propylene) | Propyne (methylacetylene) | Cyclopropane | Propadiene (allene) |
| 4 | Butane | Butene (butylene) | Butyne | Cyclobutane | Butadiene |
| 5 | Pentane | Pentene | Pentyne | Cyclopentane | Pentadiene (piperylene) |
| 6 | Hexane | Hexene | Hexyne | Cyclohexane | Hexadiene |
| 7 | Heptane | Heptene | Heptyne | Cycloheptane | Heptadiene |
| 8 | Octane | Octene | Octyne | Cyclooctane | Octadiene |
| 9 | Nonane | Nonene | Nonyne | Cyclononane | Nonadiene |
| 10 | Decane | Decene | Decyne | Cyclodecane | Decadiene |
The names used for the components used in making aviation gasoline come from the IUPAC[66] Rules for alkane nomenclature.[67]
The prefixes di, tri, tetra etc., designate several groups of the same kind. Do not consider these prefixes when alphabetizing.
Some of the chemical names include a suffix that indicates that the chemical is an isomer.[68] Isomers are compounds with the same molecular formula, but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. This group includes chain isomerism where hydrocarbon chains have variable amounts of branching. A simple example of an isomer is propanol, one of the members of the alcohol group, as indicated by its suffix "ol": it has the formula C3H8O (or C3H7OH) and occurs as two isomers: propan-1-ol or n-propyl alcohol and propan-2-ol, or isopropyl alcohol. Note that the position of the oxygen atom differs between the two: it attaches to an end carbon in the first isomer and to the center carbon in the second. The atomic arrangement determines how these fuel molecules join to one another, and how well joined they are. The fuel’s available heat in the is then determined by the mixture of carbon and hydrogen atoms, and the amount of available heat indicates how much work can be done through combustion and the resulting generation of expanding gasses in the engine cylinder. Aliphatic molecules have either a straight or branched carbon chain.
Pentane (C5H12)
Pentane is an alkane with five carbon atoms and twelve hydrogen atoms. Pentane means exclusively the n-pentane (or straight chain) isomer; the other two are "methyl butane" and "dimethyl propane."[69]
 |
| Fig. 15. Pentane (C5H12) |
Isopentane (C5H10)
Isopentane also known as methyl butane or 2-methylbutane is a branched-chain alkane with five carbon atoms and ten hydrogen atoms.[70]
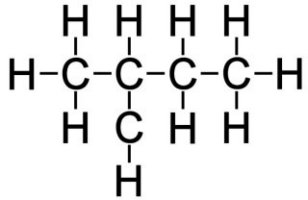 |
| Fig. 16. Isopentane (C5H10) |
Aromatics
Aromatics contain one or more benzene rings. Their name comes from the fact that many of these molecules have strong, pungent aromas.
 |
| Fig. 17. Benzene (C6H6) |
Benzene molecules have six carbon atoms joined in a ring. Half of the bonds are covalent, or double bonds, as indicated in Figure 17 by double lines between every other carbon atom. There is one hydrogen atom attached to each carbon atom in the ring.[71]
Hydrocarbons used as fuels have at least one, or as many as ten carbon atoms in each molecule and the number of hydrogen atoms vary, thereby creating a very large number of combinations. Those molecules with five or less carbon atoms are normally gasses at standard air pressure and temperature (59° F and 50% humidity). Those with six or more carbon atoms are normally liquids under the same conditions. If there are 15 to 24 carbon atoms in a hydrocarbon molecule, the liquid will partially solidify, and if 25 or more atoms are present, it will result in complete solidification.[72] The boiling point for each hydrocarbon chain increases as the carbon chain changes by adding carbon and its attached hydrogen atoms. For example, unless kept under pressure, alkanes such as methane through butane will quickly boil at room temperature, forming a gas. The next longest group pentane through hexadecane are liquids at standard room temperature, and are used as fuels and fast drying solvents, some for example, are used in dry cleaning of clothes. Next are pentadecane through hentriacontane, the oils, greases and waxes, followed the hard solids,such as asphalt. Hydrocarbon chains may be hundreds of carbon atoms long, bonding together in an almost endless variety.[73]
The vapor pressure of a liquid fuel is an indication of how easily it evaporates. Although an engine fuel must vaporize, it must also combust. Many liquids have an acceptable vapor pressure and combustibility. These liquids are typically hydrocarbons. A hydrocarbon is a molecule composed of a number of hydrogen and carbon atoms found in several different arrangements. A hydrocarbon molecule is a single unit of the combined atoms. A hydrocarbon chain is a number of hydrocarbon molecules joined to one another in various combinations, as determined by their atomic structure.[74]
In paraffinic hydrocarbons (also called alkanes), all of the carbon atoms are connected to one another in a continuous series, called a straight chain. Of all of the hydrocarbon fuels, alkanes have the lowest octane ratings. Hydrocarbons with more complex configurations, such as aromatics, olefins and branched paraffins have much higher octane ratings. For that reason, many of the refining processes designed and used in petroleum refineries produce hydrocarbons with the more complicated configurations.
Within the different hydrocarbon fuels, the carbon atoms are in two general groups based on how the carbon atoms and hydrogen atoms connect. Some, the aliphatic group, form open-ended chains that may or may not have branches, others, the aromatics, on the other hand, form rings that may have covalent[75] bonds between two or more pairs of the carbon atoms that form the ring. Figures 18 and 19 identify the structure, name and purpose of twenty-four hydrocarbon chains.
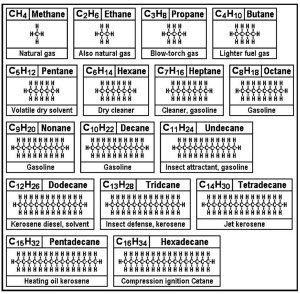 |
 |
| Fig. 18. Fuels | Fig. 19. Greases and Waxes |
Chains with more than three carbon atoms can form many different structural isomers to make rings, cubes, triangles or branches. These "isomers" have similar (but not exact, as the branched design is more easily burned) chemical properties as the straight-chain "n" forms shown. The "n" means the molecule is the base form, and not an isomer.
Crude oil is an abundant source of hydrocarbons, where decomposed organic matter provides carbon and hydrogen under conditions favorable for their formation. The same hydrocarbons are in living things. Methane (also known as swamp gas) is a product of digestion. Others are an insect attractant or form waxy coatings on some plants and by bees to make beeswax.[76]
Hydrocarbons are relatively inert (they do not change in form over time) but are easily oxidized or ignited in the oxygen found in the atmosphere. This reaction replaces the carbon-to-carbon bonds that hold the hydrocarbon together with oxygen-to-carbon bonds that produce CO (carbon monoxide) and CO2 (carbon dioxide) and the freed hydrogen atoms are absorbed by oxygen to produce H2O (water) molecules in a process called either reduction, oxidation or combustion.
Combustion
Normal combustion occurs when the fuel–air mixture ignites in the cylinder and burns at a uniform rate progressively across the combustion chamber. With properly timed ignition, maximum pressure builds up just after the piston has passed top dead center at the end of the compression stroke. The flame fronts start at each spark plug and burn in more or less wave-like forms. The type of fuel, the fuel–air ratio of the mixture, and the pressure and temperature of the fuel mixture all influence flame velocity. With normal combustion, the flame travels about 100 feet per second. The rise of temperature and pressure within the cylinder occurs at a normal rate as the fuel–air mixture burns.[77]
Combustion of a fuel is an event where the air and fuel decompose into their elemental forms. They first become a gas, and then under heat and pressure, recombine into new molecules as the gas cools. The values for the X, Y and Z variables in the following reaction represent the amount of hydrocarbon and oxygen in the fuel mixture, and will vary with the type of fuel and with the fuel to air ratio. The variable "g" represents the amount of surplus oxygen that remains after combustion. Figure 20 depicts the formula for enthalpy of combustion.
CXHYOZ(s) + (2X+Y / 2-Z ) ÷ X CO2(g) + Y H2O(1)
2 O2(g)
Fig. 20. Enthalpy of Combustion
After decomposition, the carbon and oxygen atoms combine to produce carbon dioxide (CO2) and hydrogen with oxygen combine to make water (H2O). Incomplete combustion also creates carbon smoke (C) and carbon monoxide (CO). When the nitrogen in the air combines with one or more oxygen atoms, it produces oxides of nitrogen (NOx).[78] The heat from the combustion greatly increases the volume of gas, creating the force that pushes the piston down. That force is the indicated mean effective pressure (IMEP) that, when multiplied by the piston area is the pounds of pressure exerted on the crankshaft, thereby producing torque that ultimately turns the propeller. Common fuels used in internal combustion are hydrocarbon molecules with between six and twelve carbon atoms in each molecule, have between 80% and 95% Hydrogen and between 5% and 20% carbon. Figure 21 shows characteristics of various fuels.[79] Figure 21 shows the characteristics of various fuels.
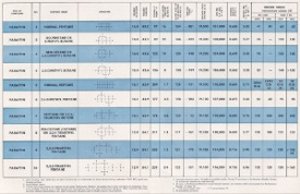 |
| Fig. 21. Characteristics of Various Fuels (1.3M PDF) |
Combining the various hydrocarbons raised the Mean Effective Pressure (MEP) at the cost of overheating the engine and the self-ignition of the fuel, called "knocking." There were many experiments on ways of improving the performance of an engine yet, at the same time, preventing detonation, the secondary ignition of the fuel–air mixture as the piston is traveling on its down-stroke after ignition. The shock waves generated by the detonation were of very high speed as they strike the cylinder walls, inducing vibrations within the engine block, which resonated at audible frequencies.[80]
This secondary ignition is spontaneous, and may be the result of the heat and pressure of the normal combustion process. One study found that the knocking happened after the initial flame front had passed through all of the volume of the combustion chamber.[81] This detonation or knocking was the limiting factor in the development of the very high horsepower engines needed for fighter and bomber aircraft in World War II. Fuels were developed and tested to increase the engine performance by reducing the tendency of the fuel to autoignite to combat preignition and detonation.
New Fuels
In World War II, there was a major effort to increase the continuous power of the aviation engines, rather than just for short periods allowed by the use of anti-demolition fluids. Increasing the octane of the fuel had dramatic effects on engines that utilize the fuel. A typical modification was to raise the intake manifold boost pressure, which greatly increased engine power by packing more of the fuel–air mixture into the engine cylinder.
n-Heptane is a straight-chain alkane with the chemical formula H3C(CH2) 5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale. The 100 point is a 100% iso-octane fuel. The octane number equates to the anti-knock qualities of a fuel compared to a mixture of heptane and iso-octane, and is expressed as the percentage of iso-octane in heptane. A derivative of this is listed on gasoline pumps. Gasoline with an octane rating of 100 was unable to prevent auto-ignition in high power engines. A new system introduced to compare fuels allowed octane numbers greater than 100. This system was the "Performance Number" or PN system, with PN 100 equal to the original 100 octane rating. After several years of testing various fuel mixtures, new fuels were developed with ratings well above PN 150. Tests of a number of solvents, cleaners and various hydrocarbon mixtures began during World War II. Among the mixtures were toluene, xylene, triptane, ether and several lesser-known chemicals.
NACA Fuel Tests
NACA Advance Restricted Report No. E4J05, October 1944
The Knock-Limited Performance of Fuel Blends Containing Aromatics Part I: Toluene, Ethyl Benzene and p-Xylene, by Carl L. Meyer and J. Robert Branstetter.
Knock-limited small-scale-engine tests were made of toluene, ethyl benzene, and p-xylene blended individually in various concentrations with selected base fuels. Data obtained for the aromatics to determine: (a) the blending sensitivity, (b) the lead susceptibility and (c) the sensitivity of the blends to inlet-air temperature.
NACA Advance Restricted Report No. E5A20, January 1945
The Knock-Limited Performance of Fuel Blends Containing Aromatics Part II; Isopropyl benzene, benzene, ando-xylene, by Carl L. Meyer and J. Robert Branstetter.
Knock-limited small-scale-engine tests were made of isopropyl benzene, benzene, and o-xylene blended individually in various concentrations with selected base fuels. The full-scale-cylinder data and the F-3 and F-4 ratings of blends containing 75% of a base fuel (65% S-3 plus 15% M-4) and 25% of each of the following aromatics: isopropyl benzene, benzene, and o-xylene, the final blends contained 4 ml tetraethyl lead (TEL) per gallon.
NACA Advance Restricted Report No. E5D16, 6 April 1945
The Knock-Limited Performance of Fuel Blends Containing Aromatics, Part III: 1,3,5-Trimethylbenzene, tert-butylbenzene and 1,2,4-trimmethylbenzene, by Carl L. Meyer and J. Robert Branstetter.
Knock-limited small-scale-engine tests were made of 1,3,5-Trimethylbenzene, tert-butylbenzene and 1,2,4 -trimethylbenzene blended individually in various concentrations with selected base fuels.
NACA Advance Restricted Report No. E5D16a, April 1945
The Knock-Limited Performance of Fuel Blends Containing Aromatics Part IV; Data for M-diemethylbenzene,1-ethyl-4-methylbenzene and sec-butylbenzene Together with a Summarization of Data for 12 Aromatic Hydrocarbons, by By Carl L. Meyer and J. Robert Branstetter.
Publication of knock-limited performance data obtained with several small-scale engines for blends containing nine aromatic hydrocarbons blended individually in various concentrations with selected base fuels. The present report presents similar knock-limited data on m-diethylbenzene, l-ethyl-4-methylbenzene and sec-butylbenzene. This report also includes a summarization and discussion of the relative performance of the 12 aromatics based on results of the small-scale-engine data and comparative published full-scale cylinder data. Although no relative order of rating has been definitely established, 1,3,5-trimethylbenzene, m-diethylbenzene, tert-butylbenzene, p-xylene, and l-ethyl-4-methylbenzene in most instances appeared to be the best of the 12 aromatics at fuel–air ratios of 0.10. At lean mixtures, under most of the operating conditions examined, m-diethyl benzene and tert-butylbenzene were more sensitive to changes of inlet-air temperature and additions of tetraethyl lead than were the base fuels.
NACA Advance Restricted Report No. E6C05, 1946
The Knock-Limited Performance of Fuel Blends Containing Aromatics Part V; n-propelbenzene, n-butylbenzene, isobutylbenzene, m-xylene, and 1-isopropyl-4-methylbenzene, by Carl L. Meyer and J. Robert Branstetter.
Knock-limited tests were made of n-propelbenzene, n-butylbenzene, isobutylbenzene, m-xylene, andl-isopropyl-4-methylbenzene blended individually in various concentrations with selected base fuels.
The five aromatics in most instances rated in the following order of decreasing antiknock effectiveness in leaded blends at a fuel–air ratio of 0.10: m-xylene, l-isopropyl-4-methylbenzene, n-propelbenzene, isobutylbenzene, and n-butylbenzene. At lean mixtures the aromatics, with the exception of n-butylbenzene, which had the lowest response at most conditions were comparable as antiknock blending agents.
Real World Results
Present day aviation fuels are the result of years of experimentation, and continue changing as necessary to improve combustion. The increase in operating altitude, economy and power requirements caused an expansion in fuel research and engine tests under real and simulated flight conditions. Along with changes in aviation fuels, there were also changes in the engines.
An engine’s sole purpose is to convert fuel into power, as a means of producing work. In propeller driven aircraft, the work is the torque transmitted to the propeller. As it rotates, the propeller accelerates air passing through its arc toward the rear of the aircraft, producing an equal, but opposite, reaction force in the forward direction to the propeller. That transmitted force moves the aircraft forward. Thrust is the forward force created by the reaction of the propeller to the accelerated.[85] In the case of a turbine driven aircraft, thrust is the result of the reaction force generated within the combustion chambers as the combustion of the fuel and air mixture generates a large amount of heat that expands the air within the combustion chamber. The force of the expanding air is equal in all directions. The heated air leaving through the combustion chamber’s exhaust area generates an opposite reaction force toward the front of the engine. The turbine fuel control is a topic included in a future article.
The Engine as an Air Pump
An engine is nothing more than an air pump with a power source built into it.[86] The engine draws air into the cylinders the volume of the cylinder increases with the rotation of the crankshaft, pulling the piston downward on the intake stroke. The amount of air drawn in is seldom equal to the cylinder displacement, as the volumetric efficiency is less than 100% due to frictional losses in the airflow and the volume of the residual combustion products that remain in the combustion chamber from the previous power cycle.[87] One other consideration is that for every eight pounds of air drawn in, there is one pound of fuel mixed with it.
What Is a Pound of Air?
How much air is there in a pound? At sea level, air weighs 0.0765 lb/ft³, or 7.65 lb per 100/ft³. When measured at a higher altitude, the air density is lower. For example, at 5,000 feet 100 ft³ of air weighs 6.59 lb.[88] Refer to the following table to see the effect of altitude on the weight of air:[89]
| Altitude | Lb/100 ft3 | Ft3 = 1 lb |
|---|---|---|
| Sea level | 7.65 | 100.00 |
| 5,000 | 6.59 | 116.09 |
| 10,000 | 5.65 | 135.40 |
| 15,000 | 4.82 | 158.71 |
| 20,000 | 4.08 | 187.50 |
| 25,000 | 3.43 | 223.03 |
| 30,000 | 2.86 | 267.48 |
| 35,000 | 2.37 | 322.78 |
| 40,000 | 1.87 | 409.09 |
A supercharger or a turbocharger helps solve the altitude problem by drawing in more air than the engine would normally be capable of if there was no supercharger or turbocharger. A supercharger or the turbocharger compresses the air in the intake manifold to the density and pressure to that found at a lower altitude.[90] The supercharger and turbocharger are topics that will be included in future articles.
Endnotes
[60] The Aircraft Engine and its Operation, p 73.
[61] Vee’s For Victory, pp 12-13.
[62] IUPAC Compendium of Chemical Terminology Gold Book, p 1596.
[63] IUPAC Compendium of Chemical Terminology Gold Book, p 227.
[64] IUPAC Compendium of Chemical Terminology Gold Book, p 56.
[65] IUPAC Compendium of Chemical Terminology Gold Book, p 369.
[66] International Union of Pure and Applied Chemistry.
[67] IUPAC Nomenclature Naming Organic Compounds.
[68] IUPAC Compendium of Chemical Terminology Gold Book, p 784.
[69] http://www.isocinfo.com
[70] http://www.isocinfo.com
[71] http://www.isocinfo.com
[72] http://www.isocinfo.com
[73] http://www.isocinfo.com
[74] Development of Aviation Fuels, p 618.
[75] IUPAC Compendium of Chemical Terminology Gold Book, p 1596.
[76] http://www.isocinfo.com
[77] Engine Conditioning For Reciprocating Engines, p 11.
[78] The John Zink Combustion Handbook, pp 60-61.
[79] http://www.isocinfo.com
[80] NACA Technical Report No. 655, p. 258.
[81] NACA Technical Report No. 655, p. 258.
[82] Universal Test Engine
[83] Vee’s For Victory, p 360.
[84] Development of Aviation Fuels, pp 621-628.
[85] Aircraft Carburetion, p 42.
[86] Aircraft Carburetion, p 43.
[87] Textbook of Aero Engines, p 1.9
[88] Aircraft Carburetion, p 43.
[89] Aircraft Carburetion, p 90.
[90] Aircraft Carburetion, p 98.